


- Stock: In Stock
- Model: 179537
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Sumamed of the tab. of p/o of 500 mg No. 3
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Drug "Sumamed ® " is applied at the infections caused by the microorganisms sensitive to azithromycin:
- ENT organs (bacterial pharyngitis / tonsillitis, sinusitis, average otitis);
- respiratory infection (bacterial bronchitis, community-acquired pneumonia);
- an infection of leather and soft tissues: the migrating erythema (initial stage of a disease of Lyme), an ugly face, impetigo, secondary pyodermatoses, an acne vulgaris (eels ordinary) moderate severity;
- an infection, sexually transmitted: the uncomplicated genital infections caused by Chlamydia trachomatis .
Structure
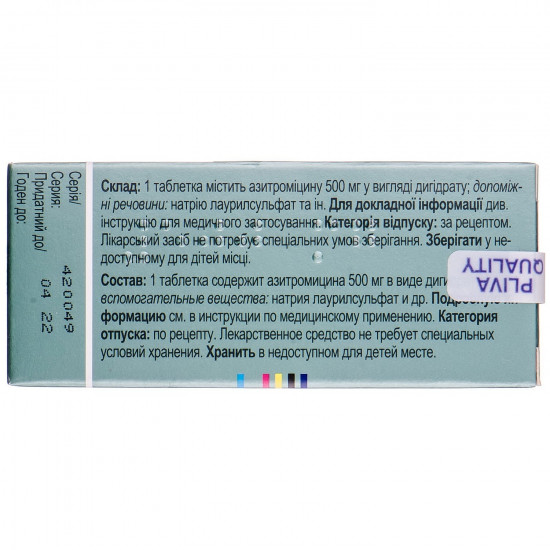
1 tablet contains azithromycin (active ingredient) of 500 mg in the form of a dihydrate.
Excipients: calcium phosphate disubstituted waterless, a hydroksipropilmetiltsellyuloza, starch corn, the starch modified, cellulose microcrystalline, sodium lauryl sulfate, magnesium stearate, indigotin (E 132), the titan dioxide (E 171), polysorbate 80, talc.
Contraindication
Hypersensitivity to azithromycin, erythromycin, to any makrolidny or ketolidny antibiotic and also to any other component of medicine.
Route of administration
"Sumamed ® ", tablets on 500 mg, it is necessary to apply in the form of a single daily dose irrespective of meal. To swallow of tablets without chewing. In case of the admission of reception of 1 dose of medicine it is necessary to accept the passed dose as soon as possible, and the subsequent - with an interval of 24 hours.
Adults and children with body weight ≥ 45 kg.
In infections of ENT organs, airways, skin and soft tissues (except the chronic migrating erythema) the general dose of azithromycin makes 1500 mg (500 mg of 1 times a day). Duration of treatment is 3 days.
In an acne vulgaris the recommended general dose of azithromycin makes 6 g which should be accepted according to the following scheme: 1 tablet on 500 mg of 1 times a day within 3 days, then - 1 tablet on 500 mg once a week within 9 weeks. A dose of the second week it is necessary to take in seven days after the first reception a pill, and 8 subsequent doses should be accepted with intervals in 7 days.
At the migrating erythema the general dose of azithromycin makes 3 g which should be accepted according to the following scheme: 1 g (2 tablets on 500 mg once) in the first day, then - on 500 mg of 1 times a day from the 2nd to the 5th day.
In infections, sexually transmitted, the recommended dose of azithromycin makes 1000 mg (2 tablets on 500 mg once).
Feature of application
Pregnant women
Are not present adequate data on use of azithromycin to pregnant women. In researches of reproductive toxicity at animals of a teratogenic adverse effect of azithromycin on a fruit it is noted, however medicine got through a placenta. Safety of use of azithromycin during pregnancy is not confirmed. Therefore azithromycin is appointed during pregnancy, only if the advantage exceeds risk.
Feeding by a breast. It was reported, that azithromycin gets into breast milk, but corresponding and properly controlled clinical trials which would give the chance to characterize pharmacokinetics of excretion of azithromycin in breast milk, was not carried out.
Fertility. The research of fertility was conducted on rats; the indicator of pregnancy decreased after administration of azithromycin. The relevance of these data on the person is unknown.
Children
"Sumamed ® ", tablets on 500 mg, children should apply with body weight ≥ 45 kg. For this group of children it is recommended to appoint an adult dose.
Drivers
Proof that azithromycin can worsen ability to steer motor transport or other mechanisms are absent, but it is necessary to consider a possibility of development of side reactions, such as delirium, hallucinations, dizziness, drowsiness, a faint, spasms which can affect ability to steer motor transport or other mechanisms.
Overdose
Experience of clinical use of azithromycin demonstrates to what side effects which develop at reception of higher than is recommended, medicine doses, are similar to those that are observed at application of usual therapeutic doses. They can include diarrhea, nausea, vomiting, a reverse hearing loss. In case of overdose if necessary intake of activated carbon and performing the general symptomatic and supporting treatment is recommended.
Side reactions
In the following table according to a class of systems and bodies and frequencies it is specified by p the side reactions defined in clinical trials and in the period of post-marketing observation observed at application of all dosage forms of azithromycin. The side reactions registered in the period of post-marketing observation are italicized. Groups on the frequency of manifestations were determined by the following scale: very often (≥ 1/10); often (≥ 1/100 to <1/10); infrequently (≥ 1/1000 to <1/100); seldom (≥ 1/10000 to <1/1000), it is very rare (<1/10000); it is unknown (it is impossible to determine by the available data). Within each group on the frequency of manifestations the undesirable phenomena are specified as reduction of their weight.
Side reactions perhaps or possibly connected with azithromycin, on the basis of the data obtained during clinical trials and during post-marketing observation.
| Class of systems and bodies | to Side reaction | to Frequency |
| Infection and an invasion | to Candidiasis, vaginal infections, pneumonia, a fungal infection, a bacterial infection, pharyngitis, a gastroenteritis, breath dysfunction, rhinitis, oral candidiasis | to infrequently |
| Pseudomembranous colitis | does not know to
| |
| from blood and lymphatic system | Leukopenia, a neutropenia, an eosinophilia | infrequently |
| Thrombocytopenia, hemolytic anemia | is unknown | |
| from the immune system | Quincke's disease, reaction of hypersensitivity | to infrequently |
| Anaphylactic reaction | does not know to
| |
| from a metabolism | Anorexia | infrequently |
| from mentality | Nervousness, insomnia | infrequently |
| Agitation | is rare | |
| Aggression, alarm, a delirium, hallucinations | does not know to
| |
| from nervous system | Headache | is frequent |
| Dizziness, drowsiness, a dysgeusia, paresthesias | infrequently | |
| Faint, spasms, a hypesthesia, psychomotor superactivity, an anosmia, an ageusia, a parosmiya, a myasthenia gravis | does not know to
| |
| from organs of sight | Disorder of vision | infrequently |
| from organs of hearing | Disorder from organs of hearing, vertigo | infrequently |
| Hearing disorder, including deafness and/or a ring in ears | does not know to
| |
| from heart | Palpitation | infrequently |
| Trembling/fibrillation of ventricles (torsade de pointes), arrhythmia, including ventricular tachycardia, | to does not know lengthening of a QT interval on the ECG | |
| from vessels | Inflows | infrequently |
| Arterial hypotension | is unknown | |
| from a respiratory system | Dispnoe, nasal bleeding | infrequently |
| from a digestive tract | Diarrhea | is very frequent
|
| Vomiting, an abdominal pain, nausea | it is frequent | |
| Constipation, a meteorism, dyspepsia, gastritis, a dysphagy, an abdominal distension, dryness in a mouth, an eructation, ulcers in an oral cavity, saliva hypersecretion | to infrequently | |
| Pancreatitis, discoloration of language | does not know to
| |
| from a gepatobiliarny system | Abnormal liver function, cholestatic jaundice | it is rare |
| Liver failure (which seldom led to a lethal outcome), fulminantny hepatitis, liver necrosis | is unknown | |
| from skin and hypodermic cellulose | Rash, an itch, urticaria, dermatitis, xeroderma, a hyperhidrosis infrequently | |
| Photosensitivity, sharp generalized exanthematous pustulyoz | is rare |
|
| Stephens-Johnson's Syndrome, a toxic epidermal necrolysis, a polymorphic erythema, reaction to medicine with an eosinophilia and system symptoms | is unknown | |
| from a musculoskeletal system | Osteoarthritis, myalgia, a dorsodynia, neck pain | infrequently |
| Arthralgia | is unknown | |
| from an urinary system | Dysuria, kidney pain | infrequently |
| Acute renal failure, interstitial nephrite | is unknown | |
| from a reproductive system and mammary glands | Uterine bleeding, testicular violations | infrequently |
| General violations and local reactions | Hypostasis, an adynamy, an indisposition, fatigue, a face edema, a stethalgia, a hyperthermia, pain, peripheral hypostasis | infrequently |
| Laboratory indicators | Lowered quantity of lymphocytes, the increased quantity of eosinophils, reduced level of bicarbonate of blood, increase in level of basophiles, increase in level of monocytes, increase in level of neutrophils | is frequent |
| Raised the AsAT level, raised the AlAT level, raised bilirubin level in blood, raised urea level in blood, raised creatinine level in blood, changes of indicators of potassium in blood, increase in level of alkaline phosphatase, increase in level of chloride, increase in level of glucose, increase in level of platelets, decrease in level of a hematocrit, increase in level of bicarbonate, a sodium level deviation | infrequently | |
| Defeat and poisoning | Complication after the procedure | infrequently |
Information on side effects which, perhaps, are connected with prevention and treatment Mycobacterium Avium Complex , is based on data of clinical trials and observations during the post-marketing period. These undesirable reactions differ on type or on frequency from about what it was reported at application of high-speed dosage forms and dosage forms of long action.
Undesirable reactions which are perhaps connected with prevention and treatment of Mycobacterium Avium Complex.
| Class of systems and bodies | Side reaction | Frequency |
| from a metabolism | Anorexia | it is frequent |
| from nervous system | Dizziness, a headache, paresthesias, a dysgeusia | is frequent |
| Hypesthesia | infrequently | |
| from organs of sight | Disorder of vision | is frequent |
| from organs of hearing | Deafness | is frequent |
| Hearing disorder, a ring in ears | infrequently | |
| from heart | Palpitation | infrequently |
| from a digestive tract | Diarrhea, an abdominal pain, nausea, a meteorism, gastrointestinal discomfort, a frequent liquid chair | is very frequent |
| from digestive system | Hepatitis | infrequently |
| from skin and hypodermic cellulose | Rash, an itch is frequent |
|
| Stephens-Johnson's Syndrome, photosensitivity | infrequently | |
| from a musculoskeletal system | Arthralgia | is frequent |
| General violations and local reactions | Increased fatigue | is frequent |
| Asthenia, an indisposition | infrequently | |
Storage conditions
Drug does not demand special storage conditions. To store out of children's reach.
Expiration date - 3 years.
Specifications
| Characteristics | |
| Active ingredients | Azithromycin |
| Amount of active ingredient | 500 mg |
| Applicant | Teva |
| Code of automatic telephone exchange | J01FA10 Azithromycin |
| Interaction with food | To |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | D.O.O. PLIVA HRVATSKA. |
| Quantity in packing | 3 tablets |
| Release form | tablets for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Sumamed |
















































