




- Stock: In Stock
- Model: 181525
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Quanil solution for shouted. comment 100mg/ml fl. 30 ml
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Pharmacological properties
Pharmacodynamics. tsitikolin stimulates biosynthesis of structural phospholipids of membranes of neurons that is confirmed by data of magnetic and resonant spectroscopy. thanks to such mechanism of action tsitikolin causes functioning of such membrane mechanisms as ion-exchange pumps and receptors which modulation is necessary for normal carrying out nervous impulses. thanks to the stabilizing action on a membrane of neurons tsitikolin has antiedematous properties which promote a wet brain reabsorption.
Pilot studies showed that tsitikolin inhibits activation of some phospholipases (A1, A2, C and D), reducing education of free radicals, prevents destruction of membrane systems and keeps antioxidant protective systems, such as glutathione.
Tsitikolin keeps a stock of energy of neurons, inhibits apoptosis and stimulates synthesis of acetylcholine.
toit is Experimentally proved that tsitikolin also has preventive neurotyre-tread effect in focal ischemia of a brain.
Clinical trials showed that tsitikolin authentically raises indicators of functional recovery at patients with an acute ischemic disorder of cerebral circulation that matches delay of spread of an ischemic brain damage according to neuroimaging.
At patients with a craniocereberal injury tsitikolin is accelerated by restoration and reduces duration and intensity of a posttraumatic syndrome.
Tsitikolin increases the level of attention and consciousness, reduces severity of the cognitive and neurologic disorders connected with brain ischemia, promotes reduction of manifestations of amnesia.
Pharmacokinetics. Tsitikolin is well absorbed at oral introduction. After administration of medicament note substantial increase of level of sincaline in blood plasma. At oral administration, medicament is almost completely soaked up. Researches showed that bioavailability at peroral and in in ways of introduction almost identical.
Drug is metabolized byin intestines and a liver with formation of sincaline and cytidine.
byAfter introduction tsitikolin it is widely distributed in structures of a brain with fast inclusion of fraction of sincaline in structural phospholipids and fractions of cytidine in cytidine nucleotides and nucleic acids. In a head brain tsitikolin it is built in cellular, cytoplasmic and mitochondrial membranes, participating in creation of fraction of phospholipids.
Only the insignificant quantity of a dose is defined byin urine and Calais (less than 3%). About 12% are removed through FROM 2 expired air. During removal of medicament with urine allocate two phases: the first phase — during 36 h in which clearance rate decreases quickly and the second phase in which clearance rate decreases much more slowly. The same staging is observed at removal through airways. Clearance rate with FROM 2 decreases quickly approximately during 15 h, then — decreases much more slowly.
Indication
Stroke, sharp phase of disturbances of cerebral circulation and their neurologic consequences. craniocereberal injury and its neurologic consequences. cognitive disturbances and behavior disorders owing to chronic vascular and degenerative cerebral disorders.
Use
Recommended dose makes 500–2000 mg/days (1–4 tablets, 5–20 ml) depending on weight of symptoms and a condition of the patient.
Drug in the form of solution which is previously mixed with a small amount of water is accepted by means of a measured glass. It is necessary to wash a measured glass with water after each use. For measuring off of doses it is possible to use the plastic pink syringe without needle.
Dose of medicament and term of treatment are established by the doctor.
toto Patients of advanced age does not need dose adjustment.
Contraindication
povyshennaya sensitivity to a tsitikolin or other components of drug; the raised tone of parasympathetic nervous system.
Side effects
from mentality: hallucinations, excitement, insomnia.
from nervous system: profound headache, dizziness, tremor.
from a cardiovascular system: AG or hypotension, tachycardia.
from a respiratory system: dispnoe.
from digestive system: nausea, vomiting, stomach ache, hypersalivation, minor change of indicators of function of a liver, incidental diarrhea.
from the immune system: allergic reactions, including rash, an itching, a Quincke's disease, an acute anaphylaxis, reddening, a small tortoiseshell, a dieback, a purpura.
General disturbances: fever, fervescence, feeling of heat, tremor, hypostasis.
Special instructions
Drug in the form of tablets contains lactose. if at the patient the intolerance of some sugars is established, it is necessary to consult with the doctor before taking this drug.
Drug in the form of solution contains Ponso dye 4R which can cause allergic reactions, an asthmatic attack, especially in patients with hypersensitivity to acetylsalicylic acid. If the intolerance of some sugars is established, it is necessary to consult with the doctor before taking this medicament as medicament contains sorbite solution. Methylparaben and propilparagidroksibenzoat, contained in composition of drug, allergic reactions can cause (perhaps, slowed down).
Use during pregnancy and feeding by a breast. Sufficient data on use of a tsitikolin for pregnant women are absent. Tsitikolin it is not necessary to apply during pregnancy, except emergency cases. During pregnancy to appoint medicine only when the expected therapeutic advantage exceeds potential risk. Data on penetration of a tsitikolin into breast milk and its action on a fruit are unknown.
Children. Experience of use of medicament for children is limited therefore it is only necessary to appoint medicine when the expected advantage exceeds any potential risk.
Ability to influence speed of response at control of vehicles or other mechanisms. In individual cases some side reactions from central nervous system can affect ability to run vehicles or to work with mechanisms.
Interaction
Tsitikolin enhances effect of a levodopa. it is not necessary to apply along with the medicines containing Meclofenoxatum.
OverdoseCases of overdose are noted by
. Storage conditions
In original packing at a temperature not above 25 °C. not to freeze and not to cool solution. in the course of storage the emergence of easy opalescence which disappears when keeping medicament at the room temperature (≈20 °C) is possible. after opening of a bottle medicament to store no more than 4 weeks
Specifications
| Characteristics | |
| Active ingredients | Tsitikolin |
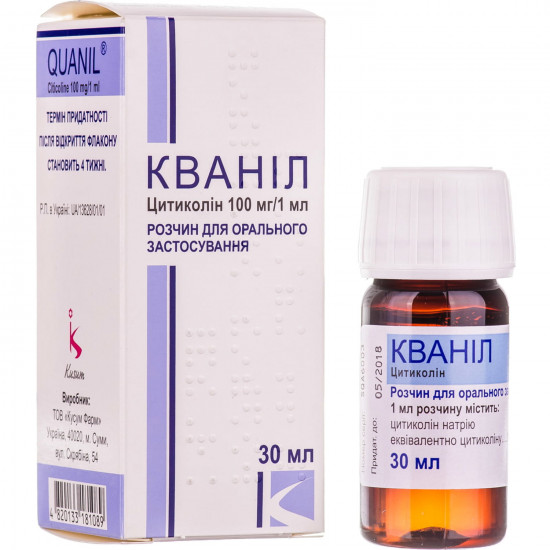
| Amount of active ingredient | 100 mg/ml |
| Applicant | Gledfarm |
| Code of automatic telephone exchange | N06BX06 Tsitikolin |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Biological |
| Prescription status | According to the prescription |
| Primary packing | bottle |
| Producer | KUSUM OF PHARMACEUTICAL LTD COMPANY |
| Quantity in packing | 30 ml |
| Release form | solution for internal use |
| Route of administration | Oral |
| Sign | Domestic |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Quanil |













![Wireless Samsung Wireless Charger Stand charger [LO] with TA 12W Black Wireless Samsung Wireless Charger Stand charger [LO] with TA 12W Black](https://www.martnets.com/image/cache/catalog/goods/my/4632/33_1500-250x250w.png)
![Wireless Samsung Wireless Charger Stand charger [LO] with TA 12W Black Wireless Samsung Wireless Charger Stand charger [LO] with TA 12W Black](https://www.martnets.com/image/cache//catalog/goods/my/gallery/4632/33/898408_middle-250x250w.png)





























