- Stock: In Stock
- Model: 184300
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Meme of the tab. of p/o of 10 mg No. 30
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Pharmacological properties
Pharmacodynamics. in manifestations of symptoms and progressing of neurodegenerative dementia an important role is played by disturbance of glutamatergichesky neurotransmission, especially with participation n-methyl-d-aspartate (nmda) - receptors.
Miemangting — potentsialzavisimy, average affinity the noncompetitive antagonist of NMDA receptors. Miemangting regulates effects patholologically of the increased level of a glutamate which can lead to dysfunction of neurons.
byaccording to researches, at patients with Alzheimer's disease of average and heavy degree after use of a memantin in a dose of 20 mg for 6 months observed such positive effects as stabilization or improvement of a condition of the general and functional sphere, cognitive opportunities.
Pharmacokinetics. Absorption. The absolute bioavailability of a memantin is about 100%. The C max is reached during 3–8 h. Signs of influence of meal on absorption are not revealed.
Distribution. Reception of a daily dose of 20 mg leads to formation of stable concentration of medicament in blood plasma within 70–150 ng/ml (0.5-1 µmol) with considerable individual variations. When assigning daily doses of 5-30 mg the relation of content of medicament is equal in cerebrospinal liquid and blood plasma to 0.52. About 45% of a memantin contact proteins of blood plasma.
Biotransformation. In a human body about 80% of a memantin circulate in the form of initial substance, the main metabolites do not show antagonistic activity in relation to NMDA. Participation of P450 cytochrome in metabolism of in vitro is not revealed.
Elimination. Miemangting eliminirutsya according to curve monoexponential dependence with T ½ 60–100 h. Volunteers with normal function of kidneys have the general clearance (Cl tot ) is 170 ml/min. / 1.73 m 2 . The renal stage of pharmacokinetics of a memantin includes also canalicular reabsorption.
Speed of renal elimination of a memantin in the conditions of alkali reaction of urine can decrease by 7–9 times. Alkalization of urine is possible as a result of sharp changes of a diet, for example upon transition from a diet rich with meat dishes on vegetarian, or owing to intensive use of antiacid gastric means.
Linearity. The pharmacokinetics has linear character in the range of doses of 10-40 mg.
Pharmakodinamichesky and pharmacokinetic communication. At use of a memantin in a dose of 20 mg/days the level of content in cerebrospinal liquid corresponds to the size k i (a braking constant) for a memantin which makes 0.5 µmol in a frontal cerebral cortex of the person.
Indication
Alzheimer's disease from moderate degree to severe forms.
Use
Treatment should be begun and carried out byunder observation of the doctor. therapy needs to be begun only in the presence of the trustee who will regularly control administration of medicament by the patient.
should take the Pill 1 time a day daily at the same time. Tablets can be applied together with food or irrespective of meal.
Adult. The maximum daily dose makes 20 mg. For the purpose of reduction of risk of emergence of side effects the maintenance dose is reached by gradual increase in a dose on 5 mg/week during the first 3 weeks according to such scheme:
1st week (1-7th day): to take ½ pill (5 mg/days) within a week;
2nd week (8-14th day): to take 1 pill (10 mg/days) within a week;
3rd week (15-21st day): to take 1.5 pill (15 mg/days) within a week;
since 4th week: to take 2 pill (20 mg) daily.
Recommended maintenance dose makes 20 mg/days
treatment Duration individually the doctor having experience of diagnostics and treatment of Alzheimer's disease defines. It is regularly necessary to estimate shipping and dosing of a memantin, it is better within 3 months from an initiation of treatment. Further the clinical effect of a memantin and reaction of the patient to treatment should be estimated regularly according to the existing clinical recommendations. The supporting treatment can be continued while the therapeutic effect remains favorable, and shipping of a memantin the patient — good. It is necessary to consider the possibility of the termination of treatment memantiny if signs of therapeutic effect disappear or the shipping of treatment by the patient worsens.
Patients of advanced age. On the basis of results of clinical trials the recommended dose for patients aged is more senior than 65 years makes 20 mg/days (2 tablets on 10 mg of 1 times a day) as it is stated above.
Renal failure. For patients with a renal failure of light severity (clearance of creatinine of 50-80 ml/min.) of a dose decline of medicament it is not required. Patients with a renal failure of moderate severity (clearance of creatinine of 30-49 ml/min.) should lower a daily dose to 10 mg. The dose can be raised to 20 mg/days according to the standard scheme if there are no negative reactions at least after 7 days of treatment. Patients with a renal failure of heavy degree (clearance of creatinine of 5-29 ml/min.) should lower a dose to 10 mg.
Abnormal liver function. For patients with an abnormal liver function easy or moderate severity (class A and B on Chayld's classification — I Drink) dose adjustment is not required. Use of a memantin for patients with a heavy abnormal liver function is not recommended.
Contraindication
Hypersensitivity to active ingredient or any component of drug.
Side effectsGeneral frequency of the undesirable phenomena at use of a memantin did not differ in
from that against the background of intake of placebo, and the phenomena usually had easy or moderate severity.
Most widespread side effects which were more often fixed in group accepting memantin than in group of placebo, were: dizziness, headache, constipation, drowsiness and AG.
At reception of a memantin the following side reactions are possible.
Infection: fungus diseases.
from the immune system: hypersensitivity.
from mentality: drowsiness, confusion of consciousness, a hallucination (mainly noted at patients with a severe form of Alzheimer's disease), psychotic reaktsii*.
from nervous system: dizziness, balance disturbance, disturbance of gait, convulsive attacks.
from heart: heart failure.
from vessels: AG, venous thrombosis / tromboembolizm.
from a respiratory system: asthma.
from digestive system: constipation, vomiting, pancreatitis.
from a gepatobiliarny system: increase in indicators of function of a liver, hepatitis.
General disturbances: headache, increased fatigue.
Alzheimer's disease is associated with a depression, the suicide ideas and a suicide. About these cases it was also reported at use of a memantin.
* Separate messages at medical use.
Special instructions
Should be careful when prescribing medicament to patients with epilepsy, patients with episodes of spasms in the anamnesis and also to patients with risk factors of developing epilepsy.
Should avoid simultaneous use of NMDA antagonists, such as amantadin, ketamine or dextromethorphan. These connections influence the same system of receptors, as well as memantin, therefore the side effects (which are generally connected with central nervous system) can be more frequent or more expressed.
Some factors causing increase in pH of urine can cause need of careful observation of the patient. The specified factors include sharp changes in a diet, for example replacement of a diet rich with meat dishes on vegetarian, or intensive use of antiacid gastric means. Besides, rn urine can raise owing to the tubular renal acidosis or heavy infections of an uric path caused by Proteus bacteria.
At the majority of clinical trials the patients who recently had a myocardial infarction and patients with dekompensirovanny stagnant heart failure (the III-IV degrees according to classification of NYHA) and also uncontrollable AG, were eliminated participants. Thereof there are only limited relevant data, and for patients with such diseases the careful observation is necessary.
Drug contains lactose therefore to patients with rare hereditary intolerance of a galactose, a lactose intolerance or disturbances of absorption of glucose galactose it is impossible to apply it.
Use during pregnancy and feeding by a breast. There are no clinical data on influence of a memantin at use it during pregnancy. Pilot studies on animals indicate a possibility of delay of pre-natal growth at influence of the concentration identical or are slightly higher applied at the person. The potential risk for the person is unknown. Miemangting it is not necessary to apply during pregnancy, except for the cases caused by accurate and obvious need.
Does not know towhether there is excretion of a memantin in breast milk, however it is possible, considering lyophilic property of substance. The women applying memantin should refrain from feeding by a breast.
Children. Not to use medicament at children in connection with insufficiency of data on safety and efficiency.
Ability to influence speed of response at control of vehicles or work with other mechanisms. Alzheimer's disease from moderately severe to severe forms usually causes deterioration in ability to drive the car and to work with mechanisms. Moreover, memantin has insignificant or moderate impact on the speed of response of the person therefore ambulatory patients should be warned about need of respect for extra care at control of vehicles or work with mechanisms.
Interaction
Should avoid simultaneous use of nmda-antagonists (amantadin, ketamine or dextromethorphan) because of risk of pharmakotoksichesky psychosis. the specified substances influence the same system of receptors, as memantin therefore the side effects (which are generally connected with central nervous system) can be noted more often or to be more expressed. there are published data on cases of possible risk at the combined use of a memantin and Phenytoinum.
Mechanism of action provides possible strengthening of effects of L-dopy, dofaminergichesky agonists and anticholinergics at simultaneous use of such NMDA antagonists as memantin. Decrease in effects of barbiturates and neuroleptics is possible. Co-administration of a memantin and antispasmodics, a dantrolen or Baclofenum can modify their effects, cause need for correction of doses.
Other medicines, such as Cimetidinum, ranitidine, procaineamide, quinidine, quinine and nicotine which use the same cationic transport system of kidneys as memantin, can also interact with memantiny, causing potential risk of increase in level of content in blood plasma.
At the combined reception of a memantin with a hydrochlorothiazide or any combined medicament containing a hydrochlorothiazide the decrease in level of keeping of the last in blood plasma is possible.
bynoted separate cases of increase in the international normalized relation (INR) at use of a memantin for the patients accepting warfarin. Though the causal relationship is not established, careful monitoring of a prothrombin time or MNO at the patients who are at the same time accepting oral anticoagulants is necessary.
byduring the pharmacokinetic researches among healthy patients of essential effects of interaction of a memantin with Glyburidum/metformin, donepezil or Galantaminum it is not revealed.
Miemangting of in vitro is not CYP inhibitor 1A2, 2A6, 2C9, 2D6, 2E1, 3A, flavinsoderzhashchy monooxygenase, an epoksidgidrolaza or a sulfation.
OverdoseExperience of studying overdose is limited to
.
Symptoms. Rather considerable overdoses (200 and 105 mg/days within 3 days respectively) were or are connected with symptoms of fatigue, weakness and/or diarrhea, or had an asymptomatic course. At overdose in 140 mg or an unspecified dose disturbance symptoms from central nervous system are noted (confusion of consciousness, slackness, drowsiness, dizziness, excitement, aggression, hallucinations, gait disorders) and/or gastrointestinal disturbances (vomiting and diarrhea).
After oral administration of 2000 mg of a memantin at the patient developed a coma within 10 days, later — a diplopia and excitement. After symptomatic treatment and a plasma exchange the patient recovered without consequences.
B other case of considerable overdose after oral administration of 400 mg of a memantin at the patient disturbances from central nervous system in the form of concern, psychosis, visual hallucinations, convulsive readiness, drowsiness, a stupor and a loss of consciousness developed. The patient recovered without consequences.
Treatment. Symptomatic, specific antidote does not exist. It is necessary to apply standard clinical procedures to removal of active ingredient from an organism, for example gastric lavage, intake of activated carbon, urine reaction acidulation methods, an artificial diuresis.
in case of excessive general stimulation of central nervous system symptomatic treatment should be applied with care.
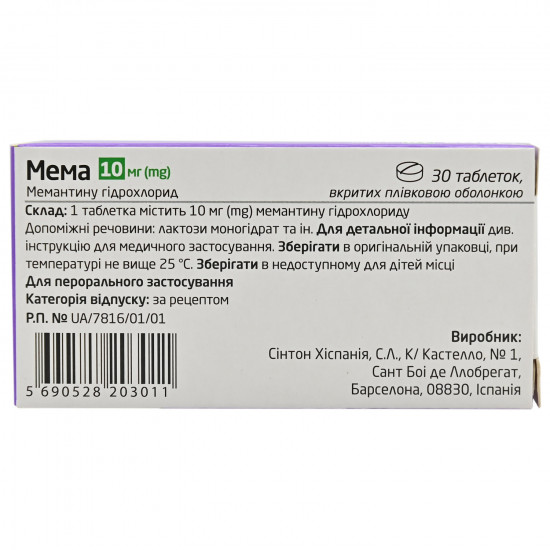
Storage conditions
In original packing at a temperature not above 25 °C.
Specifications
| Characteristics | |
| Active ingredients | Miemangting |
| Amount of active ingredient | 10 mg |
| Applicant | Teva |
| Code of automatic telephone exchange | N06DX01 Miemangting |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | HISPANIYA SYNTHON OF S.L. |
| Quantity in packing | 30 tablets (3 blisters on 10 pieces) |
| Release form | tablets for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Meme |

















































