


- Stock: In Stock
- Model: 179940
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Lindinet of 30 tab. of p/o No. 21
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
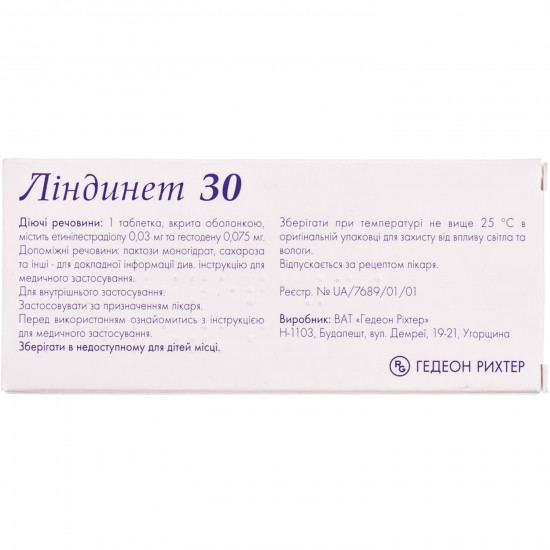
Pharmacological properties
Pharmacodynamics. lindint 20 and lindint 30 — the combined oral contraceptive medicine. blocks action of gonadotrophins. primary effect is shown by braking of an ovulation. use of medicine leads to change of characteristics of cervical slime which complicates penetration of spermatozoa into a cavity of the uterus and influences endometrium, thereby reducing a possibility of implantation of oospore. all this promotes prevention of pregnancy.
Oral contraceptives, besides contraception, have a number of positive properties.
Regulating influence on a menstrual cycle. The monthly cycle becomes regular, the frequency of a dysmenorrhea decreases. Blood loss and loss of iron at periods decreases.
Effects connected with braking of an ovulation. Frequency of appearance of functional ovarian cysts and an extrauterine pregnancy decreases.
Other effects. Frequency of developing of fibroadenomas and a fibrokist in mammary glands, inflammatory processes in bodies of a small pelvis, risk of developing of endometrial cancer decreases, the condition of skin at acne rash improves.
Pharmacokinetics. Gestoden. Absorption. Gestoden is quickly and almost completely soaked up after oral administration. The C max in blood plasma note in 1 h after reception, it makes 2–4 ng/ml. Bioavailability — about 99%.
Distribution. In blood plasma gestoden contacts albumine and globulin connecting sex hormones. About 1-2% of steroid are in a free form, 50–75% specifically contact globulin. Increasing the level of the globulin connecting sex hormones in blood ethinylestradiol increases fraction of the related gestoden and reduces its fraction connected with albumine. The average volume of distribution of a gestoden — 0.7-1.4 l/kg of body weight.
Metabolism of a gestoden is carried out byon the way characteristic of all steroids. Average plasma clearance — 0.8-1.0 ml/min. of body weight.
Discharge. Level of a gestoden in blood plasma decreases dvukhfazno. The t ½ in a terminal phase — 12–20 h
Is removed byonly in the form of metabolites: 60% — with urine, 40% — with a stake. T ½ metabolites — about 24 h
a saturation Stage. The pharmacokinetics of a gestoden depends on the level of the globulin connecting sex hormones. Concentration in blood of the globulin connecting sex hormones under the influence of ethinylestradiol increases by 3 times. At daily reception the level of a gestoden in blood plasma increases by 3–4 times and in the second half of a cycle is counterbalanced.
Ethinylestradiol. Absorption. After oral administration, ethinylestradiol is quickly and almost completely soaked up. The C max in blood plasma is reached in 1–2 h after reception and makes 30–80 pg/ml. The bioavailability because of presistemny conjugation and metabolism is about 60%.
Distribution. Ethinylestradiol completely, but nonspecific contacts albumine (about 98.5%) and induces increase in level of the globulin connecting sex hormones in blood plasma. The average volume of distribution — 5–18 l/kg of body weight.
Metabolism. Ethinylestradiol is generally metabolized by aromatic hydroxylation owing to what the hydroxylated and metilirovanny metabolites which are present at a form of free metabolites or in the form of conjugates (glucuronides and sulfates) are formed. Clearance of ethinylestradiol from blood plasma — about 5-13 ml/min. of body weight.
Discharge. Concentration in blood plasma decreases dvukhfazno. T ½ in the second phase — 16–24 h. Ethinylestradiol is removed only in the form of metabolites with urine and bile in the ratio 2:3. T ½ metabolites — about 24 h
a saturation Stage. Equilibrium concentration is established by 3-4th day of reception, and ethinylestradiol level in blood plasma is 20% higher, than after single dose.
Indication
Oral contraception.
Use
Drug should be taken within 21 days on 1 tablet a day (whenever possible at the same time). after that take a 7-day break. in the period of a 7-day break the menstrualnopodobny bleeding because of medicament withdrawal develops. usually bleeding begins for the 2nd or 3rd day after reception of the last tablet and can not end prior to reception of tablets from the following packing. next day after a 7-day break begin reception of tablets from the following packing containing 21 tablets.
First administration of drug. Lindinet 20 and Lindinet 30 should begin to be accepted from the 1st day of a menstrual cycle.
Reception of tablets can be begun withfrom the 2nd to the 5th day of periods, however in this case it is necessary to use additional non-hormonal contraceptives during the first 7 days of reception of tablets during the first cycle.
Transition to administration of medicament of Lindinet 20 or Lindinet 30 from other combined oral contraceptive. The first tablet Lindinet 20 or Lindinet 30 should be accepted after reception of the last tablet from the previous packing of other oral hormonal contraceptive medicine in the 1st day of menstrualnopodobny bleeding, but no later than one day after a break in reception of tablets (or use of placebo) from the previous packing of an oral contraceptive.
Transition to administration of medicament of Lindinet 20 or Lindinet 30 from the medicines containing only progestogen (mini-drank, injections, an implant or an intrauterine system). With mini-saw it is possible to pass to administration of medicament of Lindinet 20 or Lindinet 30 in any day of a cycle. From an implant it is possible to pass to administration of medicament of Lindinet 20 or Lindinet 30 next day after removal of an implant or an intrauterine system; after use of injection solution — in day when it is necessary to carry out the next injection, instead of an injection.
In such cases in the first 7 days needs to apply additional methods of contraception.
Administration of medicament of Lindinet 20 or Lindinet 30 after the abortion which is carried out to the I trimester of pregnancy. After abortion it is possible to take the medicament at once; in that case there is no need for application of an additional method of contraception.
Administration of medicament of Lindinet 20 or Lindinet 30 after the delivery or after the abortion which is carried out to the II trimester of pregnancy. Information on use of medicine during feeding by a breast is described in the section "Use during Pregnancy and Feeding by a Breast".
Woman which do not nurse have to begin to take the medicament in 21–28 days after the delivery or abortion in the II trimester of pregnancy. If the woman decides to take a pill later, than in 21–28 days after the delivery or abortion, then in the first 7 days it is necessary to apply additional methods of contraception.
If after the delivery or abortion, before administration of medicament it is necessary to exclude pregnancy or to wait for the first periods.
Admission of administration of drug. If having rummaged in reception there were less than 12 h, then the efficiency of medicine will not decrease. If administration of medicament was missed, the passed pill needs to be taken at once as soon as it becomes clear. The following pill from this packing should be taken in usual time.
If having rummagedin administration of medicament there were more than 12 h, its efficiency can decrease. In this case it is necessary to be guided by two basic rules:
1. Having rummaged in reception of tablets can never make more than 7 days.
2. Adequate oppression of a system a hypothalamus — a hypophysis — ovaries is reached by continuous use of medicine within 7 days.
According to it in everyday life should be guided by the recommendations below:
1st week. The woman has to take the last passed pill as soon as possible even if it is necessary to take two pill at the same time. After that she continues to take a pill in usual time. Besides, during the next 7 days it is necessary to use a barrier method of contraception, for example condom. If in the last 7 days the sexual intercourse took place, it is necessary to consider a possibility of approach of pregnancy. Than more receptions of tablets are missed and the admission is closer to a 7-day break in administration of drug, the risk of approach of pregnancy is higher.
2nd week. The woman has to take the last passed pill as soon as possible even if it is necessary to take two pill at the same time. After that she continues to take a pill in usual time. If the woman correctly took a pill within 7 days before the admission, there is no need to use additional contraceptives. Otherwise or at the admission more than one tablet are recommended to use in addition a barrier method of contraception within 7 days.
3rd week. Probability of decrease in contraceptive effect considerable because of the forthcoming 7-day break in use of medicine. However at observance of the scheme of reception of tablets it is possible to avoid decrease in contraceptive protection. If to adhere to one of the below-mentioned options, then there will be no need to use additional contraceptive resources on condition of the correct reception of a dragee for 7 days to the admission. If it not so, is recommended to adhere to the first of the options offered further and to use additional methods of contraception during the next 7 days.
1. The woman has to take the last passed pill as soon as possible even if it is necessary to take two pill at the same time. After that she continues to take a pill in usual time. Reception of tablets from new packing should be begun at once after the termination previous, that is there should not be a break in administration of drug. It is improbable that menstrualnopodobny bleeding before the end of reception of tablets from the second packing will begin though can note the smearing bloody discharges or breakthrough bleeding.
2. It is possible also to advise to stop reception of tablets from the current packing. In the second case having rummaged in administration of medicament has to make 7 days, including days of the admission of reception of tablets; administration of medicament should be begun from the following packing.
byIf missed reception of tablets and there is no menstrualnopodobny bleeding during the first usual break in administration of drug, it is necessary to consider pregnancy approach probability.
Measures taken at vomiting. If during 3–4 h after administration of medicament the vomiting begins, its active components are soaked up not completely. Such situation should be regarded as the admission of administration of medicament and to work as appropriate. If the patient does not wish to break the reception mode, the passed pill should be taken from additional packing.
Delay or acceleration of approach of periods. For a periods delay the administration of medicament should be continued from new packing without interruption. Periods it is possible to detain so, it is how necessary, up to the end of reception of the last tablet from the second packing. At a delay of periods the appearance of the breakthrough or smearing bleedings is possible. Regular administration of medicament of Lindinet 20 or Lindinet 30 can be restored after a usual 7-day break.
for the purpose of acceleration of approach of periods a 7-day break in administration of medicament is reduced by the desirable number of days. Than shorter having rummaged in administration of drug, especially it is probable that menstrualnopodobny bleeding will not come, and the breakthrough or smearing bleedings will develop at administration of medicament from the following packing.
Contraindication
Period of pregnancy or suspicion on pregnancy existence; bleedings from genitals of the obscure etiology; existence or references in the anamnesis on arterial or venous thromboembolic diseases (for example thrombophlebitis of deep veins, a pulmonary embolism, cerebrovascular violations, a myocardial infarction); existence of risk of an arterial or venous thrombembolia (violations of blood clotting, heart disease, fibrillation of auricles); existence of prodromal symptoms of thrombosis in the anamnesis (tranzitorny cerebral ischemic attack, stenocardia); cardiovascular violations (pathology of the valve(s) of heart, arrhythmia); heavy ag; presence of a benign or malignant tumor or serious illness of a liver; existence in the anamnesis of malignant tumors of a uterus or mammary glands; the diagnosed or expected malignant tumors of endometrium or other estrogenzavisimy new growths; vascular ophthalmopathy; herpes of pregnant women in the anamnesis; sickemia; lipidemia; diabetic angiopatiya; migraine with focal neurologic symptomatology; pancreatitis now or in the anamnesis if it is connected with a heavy gipertriglitseridemiya; existence in the anamnesis of cholestatic jaundice or an itch during pregnancy; progressing of an otosclerosis during the previous pregnancy; a syndrome a cudgel — Johnson, a rotor syndrome; hypersensitivity to any of medicine components.
Side effects
During the first period of administration of medicament at 10–30% of women can note such side effects: the nagrubaniye of mammary glands, deterioration in health smearing bleedings. these side effects are usually mild and through 2–4 cycles disappear.
Other possible side reactions
are possibleAt the women accepting oral contraceptives: vaginitis, delay of liquid, change of mood, headache, nausea, vomiting, acne, change of a menstrual cycle, tension of mammary glands, change of body weight and libido.
Use of oral contraceptives is connected bywith the increased risk of development of the following states:
- arterial and venous trombotichesky and tromboembolic episodes, including a myocardial infarction, a stroke, a vein thrombosis and an embolism of a pulmonary artery;
- intraepithelial neoplasia of a neck of the uterus and cervical cancer;
- breast cancer;
- benign tumors of a liver (focal nodal hyperplasia).
Infection and invasion: vulvovaginal candidiasis.
Benign, malignant and not specified new growths (including cysts and polyps): breast cancer, hepatocellular carcinoma, liver adenoma.
from blood and lymphatic system: gemolitikouremichesky syndrome.
from the immune system: anaphylactic reactions, urticaria, a Quincke's disease, heavy allergic reactions with violation of breath and circulator symptoms, aggravation of a system lupus erythematosus, aggravation of a porphyria.
Disturbance of food and metabolism: a liquid delay, decrease or increase in appetite, an abdominal distension, decrease in tolerance to glucose, a lipidemia, a gipertriglitseridemiya, ischemic colitis, inflammatory bowel disease (Crohn's disease, ulcer colitis).
Mental violations: changes of mood, depression, decrease or increase in a libido, irritability, nervousness.
from nervous system: migraine, a headache, dizziness, aggravation of a chorea, an optic neuritis (can lead to partial or full loss of sight), a stroke (LLT).
from an organ of sight: intolerance of contact lenses, eye retina artherothrombosis.
from an organ of hearing and balance: otosclerosis.
from heart: myocardial infarction.
from a cardiovascular system: AG, thrombosis, embolism.
from a digestive tract: nausea, vomiting, abdominal pain, pancreatitis.
from a liver and biliary tract: diseases of a gall bladder, cholelithiasis *, hepatocellular damage (including hepatitis and an abnormal liver function).
* Use of the combined oral contraceptives can aggravate witha course of the available disease of a gall bladder and accelerate a course of a disease at women at whom did not note disease symptoms earlier.
from skin and hypodermic cellulose: acne, hloazma (melazma of LLT), hirsutism, alopecia, knotty erythema, multiformny erythema.
from a reproductive system and mammary glands: the breakthrough bleedings smearing discharges between periods, mammary gland pain, a nagrubaniye of mammary glands.
Laboratory indicators: reduction or increase in body weight, decrease in levels of folates.
Serious and other undesirable phenomena are described byin the section SPECIAL INSTRUCTIONS.
Special instructions
Disease of the blood circulatory system. oral contraceptives increase risk of developing of a myocardial infarction. it is higher at the smoking women having accessory factors of risk (ag, a hypercholesterolemia, obesity and diabetes).
Smoking considerably increases risk of emergence of cardiovascular complications which can appear at application of oral contraceptives. This risk increases with age therefore at women 35 years are aged more senior and those which smoke much, the risk of cardiovascular complications considerably increases. The women accepting oral contraceptives are recommended to refuse smoking.
20 and Lindinet 30 should appoint Lindinet'swith care to women with risk of cardiovascular diseases.
Use of oral contraceptives increases risk of developing cerebrovascular diseases (ischemic and hemorrhagic stroke) and venous thromboembolic violations.
toIt was reported about increase in the ABP at the women accepting oral contraceptives. Increase in the ABP is more often noted at women of more advanced age and also at prolonged use.
Obtained data demonstrate that the frequency of development of AG increases depending on amount of estrogen.
should recommend to Those women at whom noted raised by the ABP or the diseases which were followed by the raised ABP or the kidneys which transferred diseases earlier other method of contraception. If, despite this, the woman with AG wishes to accept oral contraceptives, behind her state the stringent control is necessary and at substantial increase of the ABP the use of medicine should be stopped.
At most of women of the ABP after the termination of administration of medicament returns to norm, and the risk of emergence of AG further increased is uncharacteristic.
Venous and arterial thrombosis and thrombembolia. Use of the combined oral contraceptives is connected with the increased risk of venous and arterial trombotichesky and tromboembolic episodes. For each specific combination it is oestrogenic / progestagen it is necessary to apply the dosing mode which is containing the minimum quantity of estrogen and progestogen and at the same time providing the low interest of failures meeting needs of the patient.
Venous thrombosis and thrombembolia. Use of any the combined oral contraceptives (COC) results in the increased risk of the venous thromboembolic diseases (VTD).
Additional risk of development of VTZ increases in the first year of use of the PDA for the women who were not taking such medicaments yet. This risk is much lower, than risk of VTZ of pregnant women. From 100,000 pregnant women approximately at 60 note VTZ, and in 1–2% of all cases of VTZ the lethal outcome is noted.
Frequency of emergence of VTZ in the women accepting 50 mkg and less ethinylestradiol in a combination with levonorgestrel is about 20 cases on 100 thousand women a year. Frequency of emergence of VTZ in the women accepting gestoden in a combination is about 30-40 cases on 100 thousand women a year.
Risk of developing of a thrombembolia (arterial and/or venous) increases:
- with age;
- when smoking (excessive smoking and age, are especially more senior than 35 years, are accessory factors of risk);
- at the burdened family anamnesis (for example diseases of the father or the brother, the sister at young age). If there is a congenital tendency to thromboembolic diseases, it is necessary to consult to the expert before use of medicine;
- at obesity (body mass index of 30 kg/m 2 );
- at violation of exchange of fats (dislipoproteinemiya);
- at AG;
- at migraine;
- at diseases of valves of heart;
- at fibrillation of auricles;
- at a long immobilization, heavy operations, the lower extremity surgeries, severe injuries. Because the risk of thromboembolic diseases increases during the postoperative period, it is offered to stop administration of medicament for 4 weeks before operation and to begin reception in 2 weeks after the patient's remobilization.
As the period directly after the delivery is associated with the increased risk of a thrombembolia, administration of medicament of Lindinet 20 or Lindinet 30 should be begun not earlier than for the 28th day after the delivery or abortion in the II trimester of pregnancy.
Arterial thrombosis and thrombembolia. At administration of medicament of Lindinet 20 or Lindinet 30 raises risk of development of arterial trombotichesky and tromboembolic episodes. The described complications include a myocardial infarction and cerebrovascular violations (ischemic and hemorrhagic stroke, the tranzitorny ischemic attack). Risk of development of arterial trombotichesky and tromboembolic episodes higher at women with accessory factors of risk.
Should appoint bywith care Lindinet 20 or Lindinet to 30 women with risk of development of trombotichesky and tromboembolic episodes.
For the women having migraine and accepting the CPC (especially at migraine with aura), the increased risk of developing a stroke is characteristic.
Use of medicine should be stopped immediately at emergence of such symptoms of a thrombembolia: the thorax pain irradiating in the left hand, unusually the megalgia in the lower extremities, hypostases of legs pricking pain at a breath or at cough, a blood spitting.
Biochemical indicators indicating tendency to thromboembolic diseases: resistance to the activated protein C (APC), a gipergomotsisteinemiya, insufficiency of antithrombin III, protein C and protein S, existence of anti-phospholipidic antibodies (anti-cardiolipin, lupus-anticoagulant).
Tumour. In some researches it was reported about increase of cases of developing of cervical cancer at women, it is long accepting oral contraceptives, but results are ambiguous. The likelihood of developing cervical cancer depends on sexual behavior and on other factors (for example on human papillomavirus).
Cases of a breast cancer at the women accepting oral contraceptives notedclinically in earlier stage, than at the women who were not taking these drugs.
, it is long accepting hormonal contraceptives.
byestablished connection between developing of benign tumors of a liver and reception of oral contraceptives though such benign tumors reveal seldom. At a rupture of these tumors note intra belly bleeding which can lead to a lethal outcome.
At women, it is long accepting oral contraceptives, occasionally noted development of a malignant tumor of a liver.
At patients in whose anamnesis note cholestatic jaundice or the naggers during pregnancy and also at the patients who were earlier accepting the combined oral contraceptives, risk of developing these diseases is higher. In case such patients accept Lindinet 20 or Lindinet 30, careful monitoring of their state is necessary, and at a recurrence of morbid condition the use of medicine needs to be stopped.
Other states. At use of oral contraceptives the thrombosis of vessels of a retina of an eye can sometimes develop. Administration of medicament should be stopped at loss of sight (full or partial), an exophthalmos, a diplopia, at a papilledema or violations in vessels of a retina and to undergo additional medical examination.
according to researches, the relative risk of formation of gallstones increases at the women accepting the oral contraceptives or medicines containing estrogen with age. The latest researches showed that risk of developing of cholelithiasis low at use of the medicines containing hormones in a low dose.
At emergence or increase in expressiveness of attacks of migraine, emergence of a constant or repeated unusually intensive headache administration of medicament should be stopped.
Reception of the tablets Lindinet 20 or Lindinet 30 should be stopped immediately when developing an itch or at development of an epileptic seizure.
Influence on exchange of carbohydrates and lipids. At the women accepting Lindinet 20 or Lindinet 30, can note decrease in tolerance to carbohydrates. Therefore the women with diabetes taking the medicament Lindinet 20 or Lindinet 30, have to be under careful observation.
byAt some women at use of oral contraceptive revealed increase in level of triglycerides in blood. A number of progestogens reduce the LPVP level. Because estrogen increases the LPVP level XC in blood plasma, influence of the medicine Lindinet 20 or Lindinet 30 on lipid metabolism depends on balance between estrogen and progestogen and on a dose and a form of progestogen.
careful control of a condition of women with a lipidemia who, despite this, decided to accept contraceptives Is necessary for.
byAt the women with a hereditary lipidemia taking the medicament with estrogen noted sharp increase in level of triglycerides in blood plasma that could lead to development of pancreatitis.
Disturbance of a menstrual cycle. At use of the medicine Lindinet 20 or Lindinet 30, especially in the first 3 months, irregular (breakthrough) bleedings can develop. If such bleedings are present quite long time or develop after regular cycles, their reason, as a rule, non-hormonal were created; it is necessary to perform the corresponding gynecologic inspection for an exception of pregnancy or malignant new growths. If the non-hormonal reason is excluded, it is necessary to pass to intake of other medicine.
In some cases menstrualnopodobny bleeding of medicament withdrawal during a 7-day break does not develop. If to absence of bleeding the mode of use of medicine is broken or if bleeding is absent after reception of the last tablet from the second packing, before continuation of use of medicine it is necessary to exclude pregnancy.
State, demanding extra care
Medical examination. Before use of the medicine Lindinet 20 or Lindinet 30 it is necessary to collect the detailed family anamnesis and to have the general medical and gynecologic examination. These researches should be repeated regularly. At physical survey it is necessary to measure the ABP, to investigate mammary glands, to palpate a stomach, to perform gynecologic examination with a research of a cytologic smear and also laboratory researches.
needs to warn the Woman that medicine does not protect it from the infections extending sexually, in particular from AIDS.
Function of a liver. In a sharp or chronic abnormal liver function it is necessary to stop use of medicine before normalization of liver enzymes. At violation of activity of liver enzymes the metabolism of steroid hormones can be broken.
Affective disorders. It is reasonable to women at whom the depression at intake of contraceptives develops to cancel medicine and to pass temporarily to other method of contraception before definition of the cause of a depression. The women who earlier had a depression have to be under careful control, and at a recurrence of a depression it is necessary to stop use of oral contraceptive.
Level of folates. At use of oral contraceptives the level of folic acid in blood can decrease. It has clinical value only when soon after end of a course of intake of oral contraceptive there is a conception.
Hloazm's. Emergence of a hloazma is especially characteristic of women from hloazmy pregnant women in the anamnesis. To the women inclined to emergence of a hloazma, it is necessary to avoid stay in the sun and also actions of ultraviolet radiation during reception of the CPC.
Another. Except the states stated above, it is necessary to pay special attention to a condition of the woman in the presence of the following diseases: otosclerosis, multiple sclerosis, epilepsy, hysterical chorea, intermittent porphyria, tetanic states, renal failure, obesity, system lupus erythematosus, hysteromyoma.
under the influence of oral contraceptives the level of some laboratory indicators (indicators of function of a liver, kidneys, adrenal glands, a thyroid gland, fibrillation and fibrinolytic factors, lipoproteins and transport proteins) can change. Despite this, indicators remain within norm.
to Patients with rare hereditary forms of intolerance of a galactose, insufficiency of lactase or a syndrome of glyukozo-galaktazny malabsorption should not use drug.
to Patients with rare hereditary forms of intolerance of fructose, insufficiency of invertase-isomaltase or a syndrome of glyukozo-galaktozny malabsorption should not use drug.
Use during pregnancy and feeding by a breast. Prior to administration of medicament of Lindinet 20 it is necessary to exclude pregnancy.
If pregnancy occurred during use of medicine, it is necessary to stop reception of oral contraceptives immediately.
Extensive epidemiological researches did not revealincreased risk of developing of congenital malformations at the newborns who were born at the women accepting oral contraceptives before pregnancy, teratogenic action (in particular, heart disease and anomalies of development of extremities) in cases when oral contraceptives unintentionally accepted in the early stages of pregnancy.
Use of hormonal contraceptives during feeding by a breast is not recommended toas these medicines in a small amount get into milk, reduce its discharge and change structure.
Children. Drug is not used at children.
Ability to influence speed of response at control of vehicles or work with other mechanisms. Researches of rather potential impact of medicine on ability to control of vehicles or work with other mechanisms were not conducted.
Interaction between ethinylestradiol and at the same time used medicaments can lead
Interaction
to increase or decrease in level of ethinylestradiol in blood plasma. Decrease in level of ethinylestradiol in blood plasma can leadto increase in quantity of breakthrough bleedings and violations of a menstrual cycle, sometimes also note decrease in contraceptive effect of the medicine Lindinet 20 or Lindinet 30. Therefore at the simultaneous use of ethinylestradiol and medicines reducing ethinylestradiol level in blood plasma in addition to administration of medicament of Lindinet 20 or Lindinet 30 is recommended to use non-hormonal methods of contraception (for example condoms, spermitsida). If prolonged use of the medicines containing such active agents is necessary it is necessary to consider the possibility of refusal of application of hormonal contraceptives as main method of contraception.
After the termination of intake of the medicines reducing concentration of ethinylestradiol in blood is recommended to use additional non-hormonal methods of contraception at least within 7 days. After the termination of intake of the medicines capable to cause induction of microsomal enzymes of a liver and to lead to decrease in concentration of ethinylestradiol in blood plasma, it is recommended to use additional non-hormonal methods of contraception during longer period. Sometimes, depending on a dose, durations of treatment and clearance rate of the medicine which caused induction of enzymes can pass weeks before induction of enzymes of a liver completely stops.
Joint stock company
Specifications
| Characteristics | |
| Active ingredients | Gestoden, Ethinylestradiol |
| Amount of active ingredient | 0.075 mg + 0.03 mg |
| Applicant | Gideon Richter |
| Code of automatic telephone exchange | G03AA10 Gestoden and ethinylestradiol |
| Interaction with food | It doesn't matter |
| Light sensitivity | Sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | GIDEON RICHTER OF JOINT STOCK COMPANY |
| Quantity in packing | 21 tablets |
| Release form | tablets for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 30 °C |
| Trade name | Lindinet |









































